Biopharma PEG
Featured PEGs and Pharmaceutical Intermediates Manufacturer
CMO · ISO 9001:2015 Certified · Massachusetts, United States
The US FDA has approved Zynlonta (loncastuximab tesirine), the eleventh antibody-drug conjugate (ADC) and the first for ADC Therapeutics. It is the first and only CD19-targeted ADC as a single-agent treatment for adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL).
…see more
The COVID-19 pandemic has infected millions of people, but there is no obvious sign of relief due to the high infection rate, long incubation period and lack of mature treatments or vaccines. Vaccines are the most promising solution for mitigating new virus strains.
…see more
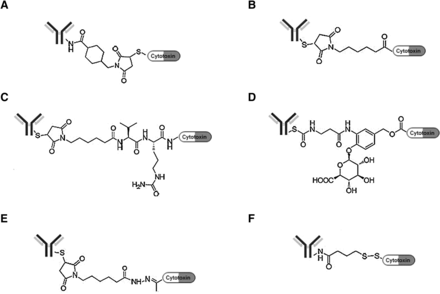
The ever-growing sophistication and variation in linker design have given rise to a new and distinct field of knowledge in synthetic chemistry. Recent developments in combination therapy, antibody-drug conjugations (ADCs), is based on linking together two compounds with different functionalities. After a decade-long trickle of ADCs, the US Food and Drug Administration approved three new ADCs in 2019 and one in 2020 to treat various cancers. That burst of activity brought the total number of FDA-approved ADCs on the market to eight, a definite uptick to end the decade.
…see more
Antibody-drug conjugates (ADCs) are a new class of targeted drugs consisting of "mAbs, cytotoxic drugs, and linkers that link the two." The ADC was originally designed to increase the effectiveness of chemotherapy and reduce its toxicity. Since the antibody is targeted (can recognize cancer cell surface antigens), the cytotoxic molecules can be selectively "transported" directly into the tumor cells to exert an anti-cancer effect while avoiding effects on healthy cells.
…see more
Antibody–drug conjugates (ADC) are one of the fastest growing anticancer drugs. This approach comprises a mAb conjugated to the cytotoxic payload via a chemical linker that directed toward a target antigen expressed on the cancer cell surface, reducing systemic exposure and therefore toxicity. ADCs are complex molecules that require careful attention to various components. Selection of an appropriate target, an mAb, cytotoxic payload, and the manner in which the antibody is linked to the payload are key determinants of the safety and efficacy of ADCs. This review provides an overview of the systemic evaluation of each component of an ADC design, improved understanding of the mechanism of action of ADC, and mechanistic pathways involved in ADC resistance and various strategies to optimize ADC design. Moreover, this review also shed light on the current status of ADCs that have gained regulatory approval from the FDA including a description of biology and chemistry, metabolic profiles, adverse events, drug interactions, and the future perspective on combination strategies with other agents, including immunotherapy.
…see more